Contact Dr. Lu for information about cancer treatments。聯繫盧博士,獲取有關癌症治療資訊。
Palm oil may up risk of obesity, diabetes, cardiovascular diseases, cancer | 棕櫚油可能會增加肥胖、糖尿病、心血管疾病、癌症的風險
Contact Dr. Lu for information about cancer treatments。聯繫盧博士,獲取有關癌症治療資訊。
Editor’s note: This is an excellent review on palm oil and its health impact. Everyone who likes processed foods should read this article. In a nutshell, palm oil is not a healthy oil. (Any added cooking oil can pose a health risk, but palm oil is worse than others such as sunflower oil, olive oil and canola oil). Palm oil is used because its unique high-melting point which is important for dry processed foods)
編者註:這是一篇關於棕櫚油及其健康影響的優秀評論。 每個喜歡加工食品的人都應該閱讀這篇文章。 簡而言之,棕櫚油不是一種健康的油。 (任何添加的食用油都會造成健康風險,但棕櫚油比葵花油、橄欖油和菜籽油等其他油更糟糕)。 使用棕櫚油是因為其獨特的高熔點對乾加工食品很重要)
Biological and Nutritional Properties of Palm Oil and Palmitic Acid: Effects on Health
1. Introduction

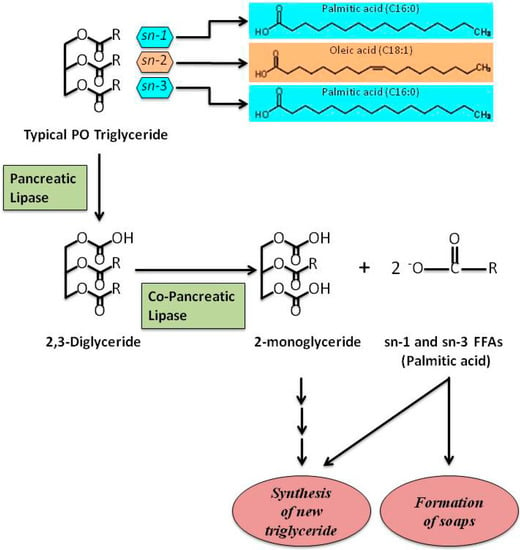
2. Palm Oil and Palmitic Acid: Role in Obesity and T2DM
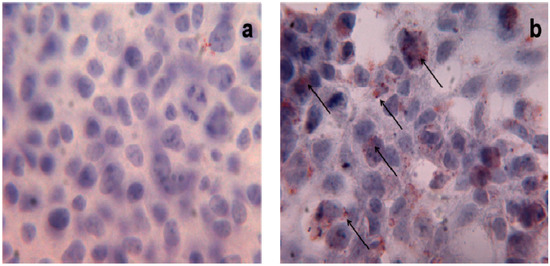
3. Palm Oil and Palmitic Acid: Role in CVD
4. Palm Oil and Palmitic Acid: Role in Cancer
PA and Cell Toxicity
5. Conclusions
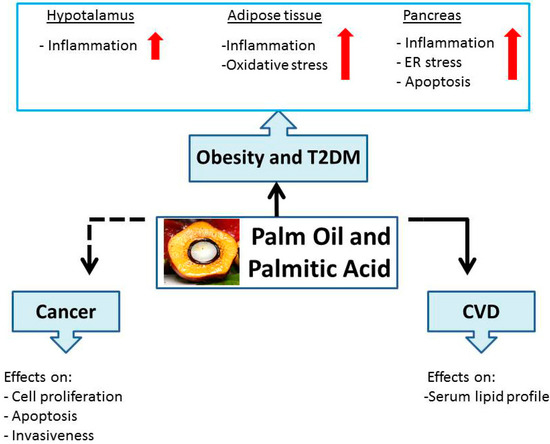
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Aranceta, J.; Perez-Rodrigo, C. Recommended dietary reference intakes, nutritional goals and dietary guidelines for fat and fatty acids: A systematic review. Br. J. Nutr. 2012, 107, S8–S22. [Google Scholar] [PubMed]
- Assmann, G.; Buono, P.; Daniele, A.; Della Valle, E.; Farinaro, E.; Ferns, G.; Krogh, V.; Kromhout, D.; Masana, L.; Merino, J.; et al. Functional foods and cardiometabolic diseases. International Task Force for Prevention of Cardiometabolic Diseases. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1272–1300. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Berger, N.A. Obesity and cancer pathogenesis. Ann. N. Y. Acad. Sci. 2014, 1311, 57–76. [Google Scholar] [CrossRef] [PubMed]
- Mba, O.I.; Dumont, M.J.; Ngadi, M. Palm Oil: Processing, characterization and utilization in the food industry. A review. Food Biosci. 2015, 10, 26–41. [Google Scholar] [CrossRef]
- Sambanthamurthi, R.; Sundram, K.; Tan, Y.A. Chemistry and biochemistry of Palm Oil. Prog. Lip Res. 2000, 39, 507–558. [Google Scholar] [CrossRef]
- Obibuzor, J.U.; Okogbenin, E.A.; Abigor, R.D. Oil recovery from palm fruits and palm kernl. In Palm Oil: Production, Processing, Characterization and Uses; Lai, O.-M., Tan, C.-P., Akoh, C.C., Eds.; AOCS Press: Urbana, IL, USA, 2012; pp. 299–328. [Google Scholar]
- Edem, D.O. Palm Oil: Biochemical, physiological, nutritional, hematological, and toxicological aspects: A review. Plant. Foods Hum. Nutr. 2002, 57, 319–341. [Google Scholar] [CrossRef] [PubMed]
- Sundram, K.; Sambanthamurthi, R.; Tan, Y.A. Palm fruit chemistry and nutrition. Asia Pac. J. Clin. Nutr. 2003, 12, 355–362. [Google Scholar] [PubMed]
- Souganidis, E.; Laillou, A.; Leyvraz, M.; Moench-Pfanner, R. A comparison of retinyl palmitate and red Palm Oil β-carotene as strategies to address Vitamin A deficiency. Nutrients 2013, 15, 3257–3271. [Google Scholar]
- Ong, A.S.; Goh, S.H. Palm Oil: A healthful and cost-effective dietary component. Food Nutr. Bull. 2002, 23, 11–22. [Google Scholar] [PubMed]
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienols in health and disease: The other half of the natural vitamin E family. Mol. Asp. Med. 2007, 28, 692–728. [Google Scholar] [CrossRef] [PubMed]
- Čmolík, J.; Pokorný, J. Physical refining of edible oils. Eur. J. Lipid Sci. Technol. 2000, 102, 472–486. [Google Scholar] [CrossRef]
- Dunford, N.T. Advancements in oil and oilseed processing. In Food and Industrial Bioproducts and Bioprocessing; John Wiley and Sons Inc.: Ames, IA, USA, 2012; pp. 115–143. [Google Scholar]
- Gunstone, F.D. Vegetable Oils in Food Technology: Composition, Properties and Uses, 2nd ed.; Blackwell Publishing Ltd.: West Essex, UK, 2011; pp. 25–133. [Google Scholar]
- Henson, I.E. A brief history of the palm oil. In Palm Oil: Production, Processing, Characterization and Uses; Lai, O.-M., Tan, C.-P., Akoh, C.C., Eds.; AOCS Press: Urbana, IL, USA, 2012. [Google Scholar]
- Gee, P.T. Analytical characteristics of crude and refined Palm Oil and fractions. Eur. J. Lipid Sci. Technol. 2007, 109, 373–379. [Google Scholar] [CrossRef]
- Jensen, R.G. The lipids in human milk. Prog. Lipid Res. 1996, 35, 53–92. [Google Scholar] [CrossRef]
- Odia, O.J.; Ofori, S.; Maduka, O. Palm Oil and the heart: A review. World J. Cardiol. 2015, 26, 144–149. [Google Scholar] [CrossRef]
- Fattore, E.; Fanelli, R. Palm oil and palmitic acid: A review on cardiovascular effects and carcinogenicity. Int. J. Food Sci. Nutr. 2013, 64, 648–659. [Google Scholar] [CrossRef]
- Fattore, E.; Bosetti, C.; Brighenti, F.; Agostoni, C.; Fattore, G. Palm oil and blood lipid-related markers of cardiovascular disease: A systematic review and meta-analysis of dietary intervention trials. Am. J. Clin. Nutr. 2014, 99, 1331–1350. [Google Scholar] [PubMed]
- Bester, D.; Esterhuyse, A.; Truter, E.J.; van Rooyen, J. Cardiovascular effects of edible oils: A comparison between four popular edible oils. Nutr. Res. Rev. 2010, 23, 334–348. [Google Scholar] [CrossRef] [PubMed]
- May, C.Y.; Nesaretnam, K. Research advancements in palm oil nutrition. Eur. J. Lipid Sci. Technol. 2014, 116, 1301–1315. [Google Scholar] [PubMed]
- Dixon, J.B. The effect of obesity on health outcomes. Mol. Cell. Endocrinol. 2010, 316, 104–108. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity and Overweight. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed on 19 January 2015).
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [PubMed]
- Exley, M.A.; Hand, L.; O’Shea, D.; Lynch, L. Interplay between the immune system and adipose tissue in obesity. J. Endocrinol. 2014, 223, R41–R48. [Google Scholar] [CrossRef] [PubMed]
- Ajuwon, K.M.; Spurlock, M.E. Palmitate activates the NF-κB transcription factor and induces IL-6 and TNF-α expression in 3T3-L1 adipocytes. J. Nutr. 2005, 135, 1841–1846. [Google Scholar] [PubMed]
- Bradley, R.L.; Fisher, F.F.; Maratos-Flier, E. Dietary fatty acids differentially regulate production of TNF-α and IL-10 by murine 3T3-L1 adipocytes. Obesity 2008, 16, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cheng, M.; Zhao, M.; Ge, A.; Guo, F.; Zhang, M.; Yang, Y.; Liu, L.; Yang, N. Differential effects of high-fat-diet rich in lard oil or soybean oil on osteopontin expression and inflammation of adipose tissue in diet-induced obese rats. Eur. J. Nutr. 2013, 52, 1181–1189. [Google Scholar] [PubMed]
- Ting, J.P.; Willingham, S.B.; Bergstralh, D.T. NLRs at the intersection of cell death and immunity. Nat. Rev. Immunol. 2008, 8, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Franchi, L.; Eigenbrod, T.; Munoz-Planillo, R.; Nunez, G. The inflammasome: A caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 2009, 10, 241–247. [Google Scholar] [PubMed]
- Jager, J.; Gremeaux, T.; Cormont, M.; Le Marchand-Brustel, Y.; Tanti, J.F. Interleukin-1β-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology 2007, 148, 241–251. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wen, H.; Gris, D.; Lei, Y.; Jha, S.; Zhang, L.; Huang, M.T.; Brickey, W.J.; Ting, J.P. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 2011, 12, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemiainduced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [PubMed]
- Stoll, L.L.; Denning, G.M.; Li, W.G.; Rice, J.B.; Harrelson, A.L.; Romig, S.A.; Gunnlaugsson, S.T.; Miller, F.J., Jr.; Weintraub, N.L. Regulation of endotoxin-induced proinflammatory activation in human coronary artery cells: Expression of functional membrane-bound CD14 by human coronary artery smooth muscle cells. J. Immunol. 2004, 173, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, S.; Witta, J.; Zhong, J.; De Villiers, W.; Eckhardt, E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J. Lipid Res. 2009, 50, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Laugerette, F.; Vors, C.; Geloen, A.; Chauvin, M.A.; Soulage, C.; Lambert-Porcheron, S.; Peretti, N.; Alligier, M.; Burcelin, R.; Laville, M.; et al. Emulsified lipids increase endotoxemia: Possible role in early postprandial low-grade inflammation. J. Nutr. Biochem. 2011, 22, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Laugerette, F.; Furet, J.P.; Debard, C.; Daira, P.; Loizon, E.; Géloën, A.; Soulage, C.O.; Simonet, C.; Lefils-Lacourtablaise, J.; Bernoud-Hubac, N.; et al. Oil composition of high-fat diet affects metabolic inflammation differently in connection with endotoxin receptors in mice. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E374–E386. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Gambino, R.; Cassader, M. Interactions between gut microbiota and host metabolism predisposing to obesity and diabetes. Annu. Rev. Med. 2011, 62, 361–380. [Google Scholar] [PubMed]
- De Wit, N.; Derrien, M.; Bosch-Vermeulen, H.; Oosterink, E.; Keshtkar, S.; Duval, C.; de Vogel-van den Bosch, J.; Kleerebezem, M.; Müller, M.; van der Meer, R. Saturated fat stimulates obesity and hepatic steatosis and affects gut microbiota composition by an enhanced overflow of dietary fat to the distal intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G589–G599. [Google Scholar] [CrossRef] [PubMed]
- Musa, C.V.; Mancini, A.; Alfieri, A.; Labruna, G.; Valerio, G.; Franzese, A.; Pasanisi, F.; Licenziati, M.R.; Sacchetti, L.; Buono, P. Four novel UCP3 gene variants associated with childhood obesity: Effect on fatty acid oxidation and on prevention of triglyceride storage. Int. J. Obes. 2012, 36, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Yu, Y.; Szabo, A.; Wu, Y.; Wang, H.; Camer, D.; Huang, X.F. Palmitic acid induces central leptin resistance and impairs hepatic glucose and lipid metabolism in male mice. J. Nutr. Biochem. 2015, 26, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Thompson, N.M.; Norman, A.M.; Donkin, S.S.; Shankar, R.R.; Vickers, M.H.; Miles, J.L. Prenatal and postnatal pathways to obesity: Different underlying mechanisms, different metabolic outcomes. Endocrinology 2007, 148, 2345–2354. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. The developmental origins of adult disease. J. Am. Coll. Nutr. 2004, 23, 588S–595S. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.P.; Guimaraes, D.E.; Mizurini, D.M.; Maia, I.C.; Ortiz-Costa, S.; Sardinha, F.L. Dietary fatty acids early in life affect lipid metabolism and adiposity in young rats. Lipids 2006, 41, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Magri, T.P.; Fernandes, F.S.; Souza, A.S.; Langhi, L.G.; Barboza, T.; Misan, V.; Mucci, D.B.; Santos, R.M.; Nunes, T.F.; Souza, S.A.; et al. Interesterified fat or palmoil as substitutes for partially hydrogenated fat in maternal diet can predispose obesity in adult male offspring. Clin. Nutr. 2015, 24, 904–910. [Google Scholar] [CrossRef]
- De Rosa, A.; Monaco, M.L.; Capasso, M.; Forestieri, P.; Pilone, V.; Nardelli, C.; Buono, P.; Daniele, A. Adiponectin oligomers as potential indicators of adipose tissue improvement in obese subjects. Eur. J. Endocrinol. 2013, 169, 37–43. [Google Scholar]
- Jiao, P.; Ma, J.; Feng, B.; Zhang, H.; Diehl, J.A.; Chin, Y.E.; Yan, W.; Xu, H. FFA-induced adipocyte inflammation and insulin resistance: Involvement of ER stress and IKKβ pathways. Obesity 2011, 19, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Kharroubi, A.T.; Darwish, H.M. Diabetes mellitus: The epidemic of the century. World J. Diabetes 2015, 6, 850–867. [Google Scholar] [CrossRef] [PubMed]
- Daniele, A.; Cammarata, R.; Pasanisi, F.; Finelli, C.; Salvatori, G.; Calcagno, G.; Bracale, R.; Labruna, G.; Nardelli, C.; Buono, P.; et al. Molecular analysis of the adiponectin gene in severely obese patients from southern Italy. Ann. Nutr. Metab. 2008, 53, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, E.J.; LeRoith, D. Obesity and Diabetes: The Increased Risk of Cancer and Cancer-Related Mortality. Physiol. Rev. 2015, 95, 727–748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K. Integration of ER stress, oxidative stress and the inflammatory response in health and disease. Int. J. Clin. Exp. Med. 2010, 3, 33–40. [Google Scholar] [PubMed]
- Kochikuzhyil, B.M.; Devi, K.; Fattepur, S.R. Effect of saturated fatty acid-rich dietary vegetable oils on lipid profile, antioxidant enzymes and glucose tolerance in diabetic rats. Indian J. Pharmacol. 2010, 42, 142–145. [Google Scholar] [PubMed]
- Storlien, L.H.; Higgins, J.A.; Thomas, T.C.; Brown, M.A.; Wang, H.Q.; Huang, X.F.; Else, P.L. Diet composition and insulin action in animal models. Br. J. Nutr. 2000, 83, S85–S90. [Google Scholar] [CrossRef] [PubMed]
- Ariyama, H.; Kono, N.; Matsuda, S.T.; Inoue, H. Decrease in membrane phospholipid unsaturation induces unfolded protein response. J. Biol. Chem. 2010, 285, 22027–22035. [Google Scholar] [CrossRef] [PubMed]
- Van Amelsvoort, J.M.; van der Beek, A.; Stam, J.J. Effects of the type of dietary fatty acid on the insulin receptor function in rat epididymal fat cells. Ann. Nutr. Metab. 1986, 30, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Kritchevsky, D.; Sundram, K. Palm oil in human nutrition: Recent advances. Asia Pac. J. Clin. Nutr. 2002, 11 (Suppl. 7), S393. [Google Scholar] [CrossRef]
- Rosqvist, F.; Iggman, D.; Kullberg, J.; Cedernaes, J.; Johansson, H.E.; Larsson, A.; Johansson, L.; Ahlström, H.; Arner, P.; Dahlman, I.; et al. Overfeeding polyunsaturated and saturated fat causes distinct effects on liver and visceral fat accumulation in humans. Diabetes 2014, 63, 2356–2368. [Google Scholar] [CrossRef] [PubMed]
- Forouhi, N.G.; Koulman, A.; Sharp, S.J.; Imamura, F.; Kröger, J.; Schulze, M.B. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: The EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol. 2014, 2, 810–818. [Google Scholar] [CrossRef]
- Yang, M.; Wei, D.; Mo, C.; Zhang, J.; Wang, X.; Han, X.; Wang, Z.; Xiao, H. Saturated fatty acid palmitate-induced insulin resistance is accompanied with myotube loss and the impaired expression of health benefit myokine genes in C2C12 myotubes. Lipids Health Dis. 2013, 12, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Simon-Szabó, L.; Kokas, M.; Mandl, J.; Kéri, G.; Csala, M. Metformin Attenuates Palmitate-Induced Endoplasmic Reticulum Stress, Serine Phosphorylation of IRS-1 and Apoptosis in Rat Insulinoma Cells. PLoS ONE 2014, 9, e97868–e97875. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wei, Y.; Wang, D.; Topczewski, F.; Passagliotti, M.J. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis indepently of ceramide in liver cells. Am. J. Physiol. Endocrinol. Metabol. 2006, 291, E275–E281. [Google Scholar] [CrossRef] [PubMed]
- Diakogiannaki, E.; Welters, H.J.; Morgan, N.G. Differential regulation of the endoplasmic reticulum stress response in pancreatic beta-cells exposed to long-chain saturated and monounsaturated fatty acids. J. Endocrinol. 2008, 197, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Mordier, S.; Iynedjian, P.B. Activation of mammalian target of rapamycin complex 1 and insulin resistance induced by palmitate in hepatocytes. Biochem. Biophys. Res. Commun. 2007, 362, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Maeda, A.; Tani, S.; Akagawa, M. Palmitate induces insulin resistance in human HepG2 hepatocytes by enhancing ubiquitination and proteasomal degradation of key insulin signaling molecules. Arch. Biochem. Biophys. 2015, 566, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, F.; Kronenberg, M.F.; Kiechl, S.; Trenkwalder, E.; Santer, P.; Oberhollenzer, F.; Egger, G.; Utermann, G.; Willeit, J. Role of lipoprotein(a) and apolipoprotein(a) phenotype in atherogenesis: Prospective results from the Bruneck study. Circulation 1999, 100, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Walldius, G.; Jungner, I.; Holme, I.; Aastveit, A.H.; Kolar, W.; Steiner, E. High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): A prospective study. Lancet 2001, 358, 2026–2033. [Google Scholar] [CrossRef]
- Walldius, G.; Jungner, I. Apolipoprotein B and apolipoprotein A-I: Risk indicators of coronary heart disease and targets for lipid-modifying therapy. J. Intern. Med. 2004, 255, 188–205. [Google Scholar] [CrossRef] [PubMed]
- Keys, A.; Menotti, A.; Aravanis, C.; Blackburn, H.; Djordevic, B.S.; Buzina, R.; Dontas, A.S.; Fidanza, F.; Karvonen, M.J.; Kimura, N.; et al. The seven countries study: 2289 deaths in 15 years. Prev. Med. 1984, 13, 141–154. [Google Scholar] [CrossRef]
- Keys, A.; Menotti, A.; Karvonen, M.J.; Aravanis, C.; Blackburn, H.; Buzina, R.; Djordjevic, B.S.; Dontas, A.S.; Fidanza, F.; Keys, M.H.; et al. The diet and 15-year death rate in the seven countries study. Am. J. Epidemiol. 1986, 124, 903–915. [Google Scholar] [PubMed]
- Kromhout, D.; Keys, A.; Aravanis, C.; Buzina, R.; Fidanza, F.; Giampaoli, S.; Jansen, A.; Menotti, A.; Nedeljkovic, S.; Pekkarinen, M. Food consumption patterns in the 1960s in seven countries. Am. J. Clin. Nutr. 1989, 49, 889–894. [Google Scholar] [PubMed]
- Menotti, A.; Keys, A.; Aravanis, C.; Blackburn, H.; Dontas, A.; Fidanza, F.; Karvonen, M.J.; Kromhout, D.; Nedeljkovic, S.; Nissinen, A.; Toshima, H.; et al. Seven countries study. First 20-year mortality data in 12 cohorts of six countries. Ann. Med. 1989, 21, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Verschuren, W.M.; Jacobs, D.R.; Bloemberg, B.P.; Kromhout, D.; Menotti, A.; Aravanis, C.; Blackburn, H.; Buzina, R.; Dontas, A.S.; Fidanza, F.; et al. Serum total cholesterol and long-term coronary heart disease mortality in different cultures. Twenty-five-year follow-up of the seven countries study. J. Am. Med. Assoc. 1995, 274, 131–136. [Google Scholar] [CrossRef]
- Mensink, R.P.; Katan, M.B. Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arterioscler. Thromb. 1992, 12, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Mensink, R.P.; Zock, P.L.; Kester, A.D.; Katan, M.B. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: A meta-analysis of 60 controlled trials. Am. J. Clin. Nutr. 2003, 77, 1146–1155. [Google Scholar] [PubMed]
- Chen, B.K.; Seligman, B.; Farquhar, J.W.; Goldhaber-Fiebert, J.D. Multi-Country analysis of palm oil consumption and cardiovascular disease mortality for countries at different stages of economic development: 1980–1997. Glob. Health 2011, 7, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Utarwuthipong, T.; Komindr, S.; Pakpeankitvatana, V.; Songchitsomboon, S.; Thongmuang, N. Small dense low-density lipoprotein concentration and oxidative susceptibility changes after consumption of soybean oil, rice bran oil, palm oil and mixed rice bran/palm oil in hypercholesterolaemic women. J. Int. Med. Res. 2009, 37, 96–104. [Google Scholar] [CrossRef]
- Truswell, A.S. Comparing palmolein with different predominantly monounsaturated oils: Effect on plasma lipids. Int. J. Food Sci. Nutr. 2000, 51, S73–S77. [Google Scholar] [CrossRef] [PubMed]
- Vega-López, S.; Ausman, L.M.; Jalbert, S.M.; Erkkilä, A.T.; Lichtenstein, A.H. Palm and partially hydrogenated soybean oils adversely alter lipoprotein profiles compared with soybean and canola oils in moderately hyperlipidemic subjects. Am. J. Clin. Nutr. 2006, 84, 54–62. [Google Scholar] [PubMed]
- Clandinin, M.T.; Cook, S.L.; Konrad, S.D.; Goh, Y.K.; French, M.A. The effect of palmitic acid on lipoprotein cholesterol levels and endogenous cholesterol synthesis in hyperlipidemic subjects. Lipids 1999, 34, S121–S124. [Google Scholar] [CrossRef]
- Clandinin, M.T.; Cook, S.L.; Konrad, S.D.; French, M.A. The effect of palmitic acid on lipoprotein cholesterol levels. Int. J. Food Sci. Nutr. 2000, 51, S61–S71. [Google Scholar] [PubMed]
- Marzuki, A.; Arshad, F.; Razak, T.A.; Jaarin, K. Influence of dietary fat on plasma lipid profiles of Malaysian adolescents. Am. J. Clin. Nutr. 1991, 53 (Suppl. 4), 1010S–1014S. [Google Scholar] [PubMed]
- Sundram, K.; Ismail, A.; Hayes, K.C.; Jeyamalar, R.; Pathmanathan, R. Trans (elaidic) fatty acids adversely affect the lipoprotein profile relative to specific saturated fatty acids in humans. J. Nutr. 1997, 127, 514S–520S. [Google Scholar] [PubMed]
- Sundram, K.; Hayes, K.C.; Siru, O.H. Dietary palmitic acid results in lower serum cholesterol than does a lauric-myristic acid combination in normolipemic humans. Am. J. Clin. Nutr. 1994, 59, 841–846. [Google Scholar] [PubMed]
- Favé, G.; Coste, T.C.; Armand, M. Physicochemical properties of lipids: New strategies to manage fatty acid bioavailability. Cell. Mol. Biol. 2004, 50, 815–831. [Google Scholar] [PubMed]
- Karupaiah, T.; Sundram, K. Effects of stereospecific positioning of fatty acids in triacylglycerol structures in native and randomized fats: A review of their nutritional implications. Nutr. Metab. 2007, 4, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Kromhout, D.; Menotti, A.; Bloemberg, B.; Aravanis, A.; Blackburn, H.; Buzina, R.; Dontas, A.S.; Fidanza, F.; Giampaoli, S.; Jansen, A. Dietary saturated and trans fatty acids and cholesterol and 25-year mortality from coronary heart disease: The Seven Countries Study. Prev. Med. 1995, 24, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.; Frost, C.; Collins, R.; Appleby, P.; Peto, R. Dietary lipids and blood cholesterol: Quantitative meta-analysis of metabolic ward studies. BMJ 1997, 314, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Renaud, S.C.; Ruf, J.C.; Petithory, D. The positional distribution of fatty acids in palm oil and lard influences their biologic effects in rats. J. Nutr. 1995, 125, 229–237. [Google Scholar]
- Kritchevsky, D.; Tepper, S.A.; Wright, S.; Kuksis, A.; Hughes, T.A. Cholesterol vehicle in experimental atherosclerosis. Cottonseed oil and randomized cottonseed oil. Nutr. Res. 1998, 18, 259–264. [Google Scholar] [CrossRef]
- Kritchevsky, D.; Tepper, S.A.; Kuksis, A.; Eghtedary, K.; Klurfeld, D.M. Cholesterol vehicle in experimental atherosclerosis. Native and randomized lard and tallow. J. Nutr. Biochem. 1998, 9, 582–585. [Google Scholar] [CrossRef]
- Kritchevsky, D.; Tepper, S.A.; Chen, S.C.; Meijer, G.W.; Krauss, R.M. Cholesterol vehicle in experimental atherosclerosis. 23. Effects of specific synthetic triglycerides. Lipids 2000, 35, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Kritchevsky, D.; Tepper, S.A.; Czarnecki, S.K.; Sundram, K. Red palm oil in experimental atherosclerosis. Asia Pacific J. Clin. Nutr. 2002, 11, S433–S437. [Google Scholar] [CrossRef]
- López-López, A.; Castellote-Bargalló, A.I.; Campoy-Folgoso, C.; Rivero-Urgël, M.; Tormo-Carnicé, R.; Infante-Pina, D.; López-Sabater, M.C. The influence of dietary palmitic acid triacylglyceride position on the fatty acid, calcium and magnesium contents of at term newborn faeces. Early Hum. Dev. 2001, 65, S83–S94. [Google Scholar] [CrossRef]
- Sczaniecka, A.K.; Brasky, T.M.; Lampe, J.W.; Patterson, R.E.; White, E. Dietary intake of specific fatty acids and breast cancer risk among postmenopausal women in the VITAL cohort. Nutr. Cancer 2012, 64, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A.M.; Williamson, E.J.; Bassett, J.K.; MacInnis, R.J.; Giles, G.G.; English, D.R. Dietary and biomarker estimates of fatty acids and risk of colorectal cancer. Int. J. Cancer 2015. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.D.; Walker, S.P.; Simpson-Smith, C.M.; Lindsay, C.M.; Smith, G.; McFarlane-Anderson, N.; Bennett, F.I.; Coard, K.C.; Aiken, W.D.; Tulloch, T.; et al. Associations of whole-blood fatty acids and dietary intakes with prostate cancer in Jamaica. Cancer Causes Control 2012, 23, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Saadatian-Elahi, M.; Norat, T.; Goudable, J.; Riboli, E. Biomarkers of dietary fatty acid intake and the risk of breast cancer: A meta-analysis. Int. J. Cancer 2004, 111, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.J.; Zhou, J.D.; Dong, J.Y.; Ding, W.Q.; Wu, J.C. Dietary intake of n-3 fatty acids and colorectal cancer risk: A meta-analysis of data from 489,000 individuals. Br. J. Nutr. 2012, 108, 1550–1556. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, I.A.; Katan, M.B.; Zock, P.L. Dietary α-linolenic acidis associated with reduced risk of fatal coronary heart disease, but increased prostate cancer risk: A meta-analysis. J. Nutr. 2004, 134, 919–922. [Google Scholar] [PubMed]
- Wolk, A.; Bergstrom, R.; Hunter, D.; Willett, W.; Ljung, H.; Holmberg, L.; Bergkvist, L.; Bruce, A.; Adami, H.O. A prospective study of association of monounsaturated fat and other types of fat with risk of breast cancer. Arch. Intern. Med. 1998, 158, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Lof, M.; Sandin, S.; Lagiou, P.; Hilakivi-Clarke, L.; Trichopoulos, D.; Adami, H.O.; Weiderpass, E. Dietary fat and breast cancer risk in the Swedish women’s lifestyle and health cohort. Br. J. Cancer 2007, 97, 1570–1576. [Google Scholar] [CrossRef] [PubMed]
- Sieri, S.; Krogh, V.; Ferrari, P.; Berrino, F.; Pala, V.; Thiébaut, A.C.; Tjønneland, A.; Olsen, A.; Overvad, K.; Jakobsen, M.U. Dietary fat and breast cancer risk in the European Prospective Investigation into Cancer and Nutrition. Am. J. Clin. Nutr. 2008, 88, 1304–1312. [Google Scholar] [PubMed]
- Thiebaut, A.C.; Kipnis, V.; Chang, S.C.; Subar, A.F.; Thompson, F.E.; Rosenberg, P.S.; Hollenbeck, A.R.; Leitzmann, M.; Schatzkin, A. Dietary fat and postmenopausal invasive breast cancer in the national institutes of health-AARP diet and health study cohort. J. Natl. Cancer Inst. 2007, 99, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Theodoratou, E.; McNeill, G.; Cetnarskyj, R.; Farrington, S.M.; Tenesa, A.; Barnetson, R.; Porteous, M.; Dunlop, M.; Campbell, H. Dietary fatty acids and colorectal cancer: A case-control study. Am. J. Epidemiol. 2007, 166, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Crowe, F.L.; Allen, N.E.; Appleby, P.N.; Overvad, K.; Aardestrup, I.V.; Johnsen, N.F.; Tjønneland, A.; Linseisen, J.; Kaaks, R.; Boeing, H.; et al. Fatty acid composition of plasma phospholipids and risk of prostate cancer in a case-control analysis nested within the European Prospective Investigation into Cancer and Nutrition. Am. J. Clin. Nutr. 2008, 88, 1353–1363. [Google Scholar] [PubMed]
- Kurahashi, N.; Inoue, M.; Iwasaki, M.; Sasazuki, S.; Tsugane, A.S. Japan Public Health Center-Based Prospective Study Group. Dairy product, saturated fatty acid, and calcium intake and prostate cancer in a prospective cohort of Japanese men. Cancer Epidemiol. Biomark. Prev. 2008, 17, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Hardy, S.; Langelier, Y.; Prentki, M. Oleate activates phosphatidylinositol 3-kinase and promotes proliferation and reduces apoptosis of MDA-MB-231 breast cancer cells, whereas palmitate has opposite effects. Cancer Res. 2000, 60, 6353–6358. [Google Scholar] [PubMed]
- Rossini, A.; Zanobbio, L.; Sfondrini, L.; Cavalleri, A.; Secreto, G.; Morelli, D.; Palazzo, M.; Sommariva, M.; Tagliabue, E.; Rumio, C.; et al. Influence of fatty acid-free diet on mammary tumor development and growth rate in HER-2/Neu transgenic mice. J. Cell. Physiol. 2013, 228, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, H.; Nair, J.; Owen, R.W. Dietary polyunsaturated fatty acids and cancers of the breast and colorectum: Emerging evidence for their role as risk modifiers. Carcinogenesis 1999, 20, 2209–2218. [Google Scholar] [CrossRef] [PubMed]
- Kolonel, L.N.; Nomura, A.M.; Cooney, R.V. Dietary fat and prostate cancer: Current status. J. Natl. Cancer Inst. 1999, 91, 414–428. [Google Scholar] [CrossRef] [PubMed]
- Escrich, E.; Solanas, M.; Moral, R.; Costa, I.; Grau, L. Are the olive oil and other dietary lipids related to cancer? Experimental evidence. Clin. Transl. Oncol. 2006, 8, 868–883. [Google Scholar] [CrossRef] [PubMed]
- Kuriki, K.; Wakai, K.; Hirose, K.; Matsuo, K.; Ito, H.; Suzuki, T.; Saito, T.; Kanemitsu, Y.; Hirai, T.; Kato, T.; et al. Risk of colorectal cancer is linked to erythrocyte compositions of fatty acids as biomarkers for dietary intakes of fish, fat, and fatty acids. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Shannon, J.; King, I.B.; Moshofsky, R.; Lampe, J.W.; Gao, D.L.; Ray, R.M.; Thomas, D.B. Erythrocyte fatty acids and breast cancer risk: A case-control study in Shanghai, China. Am. J. Clin. Nutr. 2007, 85, 1090–1097. [Google Scholar] [PubMed]
- Escrich, E.; Solanas, M.; Moral, R.; Escrich, R. Modulatory effects and molecular mechanisms of olive oil and other dietary lipids in breast cancer. Curr. Pharm. Des. 2011, 17, 813–830. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Dietary fatty acids and the immune system. Lipids 1999, 34, S137–S140. [Google Scholar] [CrossRef] [PubMed]
- Ge, G.; Wu, J.; Lin, Q. Effect of membrane fluidity on tyrosine ki-nase activity of reconstituted epidermal growth factor receptor. Biochem. Biophys. Res. Commun. 2001, 282, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Soto-Guzman, A.; Navarro-Tito, N.; Castro-Sanchez, L.; Martinez-Orozco, R.; Salazar, E.P. Oleic acid promotes MMP-9 secretion and invasion in breast cancer cells. Clin. Exp. Metastasis 2010, 27, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, T.; Adair, J.E.; Lih, F.B.; Hsi, L.C.; Rubino, M.; Eling, T.E.; Tomer, K.B.; Yashiro, M.; Hirakawa, K.; Olden, K.; et al. Elevated dietary linoleic acid increases gastric carcinoma cell invasion and metastasis in mice. Br. J. Cancer 2010, 103, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
- Kuhajda, F.P.; Pizer, E.S.; Li, J.N.; Mani, N.S.; Frehywot, G.L.; Townsend, C.A. Synthesis and antitumor activity of an inhibitor of fatty acid synthase. Proc. Natl. Acad. Sci. USA 2000, 97, 3450–3454. [Google Scholar] [CrossRef] [PubMed]
- Nigro, E.; Imperlini, E.; Scudiero, O.; Monaco, M.L.; Polito, R.; Mazzarella, G.; Orrù, S.; Bianco, A.; Daniele, A. Differentially expressed and activated proteins associated with non small cell lung cancer tissues. Respir. Res. 2015, 16, 74. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.R.; Liu, W.; Xing, F.; Fukuda, K.; Watabe, K. Anti-cancer drugs targeting fatty acid synthase (FAS). Recent Pat. Anticancer Drug Discov. 2012, 7, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Czaja, M.J. Regulation of lipid stores and metabolism by lipophagy. Cell. Death Differ. 2013, 20, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Knævelsrud, H.; Simonsen, A. Lipids in autophagy: Constituents, signaling molecules and cargo with relevance to disease. Biochim. Biophys. Acta 2012, 1821, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Cuervo, A.M. Lipophagy: Connecting autophagy and lipid metabolism. Int. J. Cell Biol. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Borradaile, N.M.; Han, X.; Harp, J.D.; Gale, S.E.; Ory, D.S.; Schaffer, J.E. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J. Lipid Res. 2006, 47, 2726–2737. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Dai, D.L.; Yao, L.; Yu, H.H.; Ning, B.; Zhang, Q.; Chen, J.; Cheng, W.H.; Shen, W.; Yang, Z.X. Saturated fatty acid induction of endoplasmic reticulum stress and apoptosis in human liver cells via the PERK/ATF4/CHOP signaling pathway. Mol. Cell. Biochem. 2012, 364, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Li, K.; Laybutt, D.R.; He, M.L.; Zhao, H.L.; Chan, J.C.; Xu, G. Bip overexpression, but not CHOP inhibition, attenuates fatty-acid-induced endoplasmic reticulum stress and apoptosis in HepG2 liver cells. Life Sci. 2010, 87, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Lee, A.Y.; Park, S.; Kim, J.H.; Cho, M.H. Multiple pathways are involved in palmitic acid-induced toxicity. Food Chem. Toxicol. 2014, 67, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Unger, R.H.; Zhou, Y.T. Lipotoxicity of beta-cells in obesity and in other causes of fatty acid spillover. Diabetes 2001, 50 (Suppl. 1), S118–S121. [Google Scholar] [CrossRef] [PubMed]
- Lambertucci, R.H.; Leandro, C.G.; Vinolo, M.A.; Nachbar, R.T.; Dos Reis Silveira, L.; Hirabara, S.M.; Curi, R.; Pithon-Curi, T.C. The effects of palmitic acid on nitric oxide production by rat skeletal muscle: Mechanism via superoxide and iNOS activation. Cell. Physiol. Biochem. 2012, 30, 1169–1180. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hirabara, S.M.; Curi, R.; Maechler, P. Saturated fatty acid-induced insulin resistance is associated with mitochondrial dysfunction in skeletal muscle cells. J. Cell. Physiol. 2010, 222, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Yuzefovych, L.; Wilson, G.; Rachek, L. Different effects of oleate vs. palmitate on mitochondrial function, apoptosis, and insulin signaling in L6 skeletal muscle cells: Role of oxidative stress. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E1096–E1105. [Google Scholar] [CrossRef] [PubMed]
- Fromenty, B.; Robin, M.A.; Igoudjil, A.; Mansouri, A.; Pessayre, D. The ins and outs of mitochondrial dysfunction in NASH. Diabetes Metab. 2004, 30, 121–38. [Google Scholar] [CrossRef]
- Nakamura, S.; Takamura, T.; Matsuzawa-Nagata, N.; Takayama, H.; Misu, H.; Noda, H.; Nabemoto, S.; Kurita, S.; Ota, T.; Ando, H.; Miyamoto, K.; et al. Palmitate induces insulin resistance in H4IIEC3 hepatocytes through reactive oxygen species produced by mitochondria. J. Biol. Chem. 2009, 284, 14809–14818. [Google Scholar] [CrossRef] [PubMed]
- Brookheart, R.T.; Michel, C.I.; Listenberger, L.L.; Ory, D.S.; Schaffer, J.E. The non-coding RNA gadd7 is a regulator of lipid-induced oxidative and endoplasmic reticulum stress. J. Biol. Chem. 2009, 284, 7446–7454. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Naseem, R.H.; Park, B.H.; Garry, D.J.; Richardson, J.A.; Schaffer, J.E.; Unger, R.H. Alpha-lipoic acid prevents lipotoxic cardiomyopathy in acyl CoA-synthase transgenic mice. Biochem. Biophys. Res. Commun. 2006, 344, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Metreveli, N.S.; Donthi, R.V.; Xia, S.; Xu, M.; Carlson, E.C.; Epstein, P.N. Catalase protects cardiomyocyte function in models of type 1 and type 2 diabetes. Diabetes 2004, 53, 1336–1343. [Google Scholar] [CrossRef]
- Listenberger, L.L.; Ory, D.S.; Schaffer, J.E. Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J. Biol. Chem. 2001, 276, 14890–14895. [Google Scholar] [CrossRef] [PubMed]
- García-Ruiz, I.; Fernández-Moreira, D.; Solís-Muñoz, P.; Rodríguez-Juan, C.; Díaz-Sanjuán, T.; Muñoz-Yagüe, T.; Solís-Herruzo, J.A. Mitochondrial complex I subunits are decreased in murine nonalcoholic fatty liver disease: Implication of peroxynitrite. J. Proteome Res. 2010, 9, 2450–2459. [Google Scholar] [CrossRef] [PubMed]
- García-Ruiz, I.; Solís-Muñoz, P.; Fernández-Moreira, D.; Muñoz-Yagüe, T.; Solís-Herruzo, J.A. In vitro treatment of HepG2 cells with saturated fatty acids reproduces mitochondrial dysfunction found in nonalcoholic steatohepatitis. Dis. Model Mech. 2015, 8, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Rizwan Alam, M.; Waldeck-Weiermair, M.; Karsten, F.; Groschner, L.; Riederer, M.; Hallström, S.; Rockenfeller, P.; Konya, V.; Heinemann, A.; et al. Inhibition of autophagy rescues palmitic acid-induced necroptosis of endothelial cells. J. Biol. Chem. 2012, 287, 21110–21120. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
