Contact Dr. Lu for information about cancer treatments。聯繫盧博士,獲取有關癌症治療資訊。
Dental Implants: What You Should Know | 種植牙:您應該知道的
Contact Dr. Lu for information about cancer treatments。聯繫盧博士,獲取有關癌症治療資訊。
Dental implants are medical devices surgically implanted into the jaw to restore a person’s ability to chew or their appearance. They provide support for artificial (fake) teeth, such as crowns, bridges, or dentures.
On this page:
- Background
- Recommendations for Patients
- Benefits and Risks
- Reporting Problems to the FDA
- Additional Resources
Background
When a tooth is lost due to injury or disease, a person can experience complications such as rapid bone loss, defective speech, or changes to chewing patterns that result in discomfort. Replacing a lost tooth with a dental implant can significantly improve the patient’s quality of life and health.
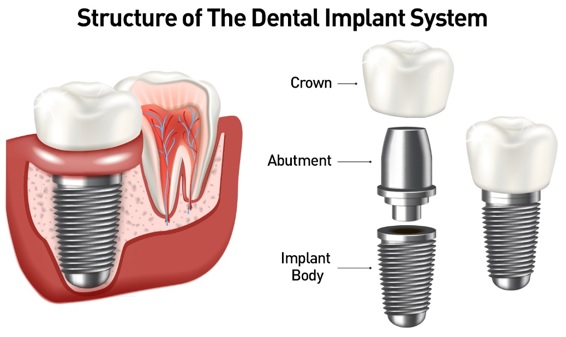
Dental implant systems consist of a dental implant body and dental implant abutment and may also include an abutment fixation screw. The dental implant body is surgically inserted in the jawbone in place of the tooth’s root. The dental implant abutment is usually attached to the implant body by the abutment fixation screw and extends through gums into the mouth to support the attached artificial teeth.
Figure 1. Structure of The Dental Implant System
Recommendations for Patients
Before choosing dental implants, talk to your dental provider about the potential benefits and risks, and whether you are a candidate for the procedure.
Things to consider:
- Your overall health is an important factor in determining whether you are a good candidate for dental implants, how long it will take to heal, and how long the implant may stay in place.
- Ask your dental provider what brand and model of dental implant system is being used and keep this information for your records.
- Smoking may affect the healing process and decrease the long-term success of the implant.
- The healing process for the implant body may take several months or longer, during which time you typically have a temporary abutment in place of the tooth.
After the dental implant procedure:
- Carefully follow the oral hygiene instructions given to you by your dental provider. Regularly cleaning the implant and surrounding teeth is very important for long-term success of the implant.
- Schedule regular visits with your dental provider.
- If your implant feels loose or painful, tell your dental provider right away.
Benefits and Risks
Dental implants can significantly improve the quality of life and the health of a person who needs them. However, complications may sometimes occur. Complications can occur soon after dental implant placement or much later. Some complications result in implant failure (usually defined as implant looseness or loss). Implant failure can result in the need for another surgical procedure to fix or replace the implant system.
Benefits of Dental Implant Systems:
- Restores the ability to chew
- Restores cosmetic appearance
- Helps keep the jawbone from shrinking due to bone loss
- Preserves the health of the surrounding bone and gums
- Helps keep adjacent (nearby) teeth stable
- Improves quality of life
Risks Associated with Dental Implant Systems:
- Damage to surrounding natural teeth during implant placement
- Injury to the surrounding tissues during surgery, such as sinus perforation
- Injury during surgery (for example, fracture of surrounding jawbone)
- Inadequate function, such as feeling like the teeth do not bite together normally
- A sensation that the tooth is loose or twisting in place resulting from an abutment screw loosening
- Implant body failure (looseness of the implant body)
- due to systemic infection, which may be more likely in patients with uncontrolled diabetes
- due to local infection in bone and gums supporting the implant body
- due to delayed healing, which may be more likely in patients who smoke
- Difficulty cleaning the gums around the implant, resulting in poor oral hygiene
- Untreated periodontal disease
- Post-surgical numbness due to nerve impingement or damage
- Always notify health care providers and imaging technicians that you have dental implants before any magnetic resonance imaging (MRI) or x-ray procedures. Dental implants can distort or interfere with these images. FDA is not aware of any adverse events reported for MRI or x-ray procedures with dental implants.
Ways Dental Implants are Evaluated for Safety
Dental implants systems are typically made of materials that follow international consensus standards of the International Organization for Standardization (ISO) or ASTM International. These standards have details of what makes a safe material. Most dental implant systems are made of titanium or zirconium oxide. Other materials such as gold alloys, cobalt-based alloys, titanium alloys, or ceramic materials are sometimes used. The safety profiles of these materials are well-known.
Dental implant systems are evaluated according to international consensus standards. Biocompatibility testing, to show that bodily contact with the device does not cause complications like irritation or allergic reaction, is part of the evaluation that helps ensure the materials in the dental implant system are safe and do not cause adverse effects when implanted in people.
For manufacturers to market dental implant systems in the United States, they must first show the FDA their systems are as safe and as effective as dental implant systems already on the market.
Reporting Dental Implant System Problems to the FDA
Prompt reporting of adverse events can help the FDA identify and better understand the risks associated with medical products. If you have problems associated with your dental implant system including the dental implant body, the dental abutment, or the dental abutment screw, we encourage you to file a voluntary report through MedWatch, the FDA Safety Information and Adverse Event Reporting program.
To help us learn as much as possible about the adverse events associated with dental implant systems, please include the following information in your report, if available:
- Date of device implantation
- Identification of dental implant system used
- Description of the problem including date of onset, and any diagnosis and follow-up treatment
- Description of medical or surgical interventions taken, including prior interventions, if any
- Pertinent medical and dental history
Additional Resources
- American Dental Association (ADA), “PatientSmart Patient Education Center: Dental Implants”External Link Disclaimer
- American Academy of Periodontology (AAP), “Dental Implants”
This is an article originally published on FDA.gov